2DG- The Magic Pill For Covid-19?
At a time where the devastating second wave of Covid-19 is sweeping across India, people is banking their hopes high on 2-DG, the indigenously developed Covid-19 drug launched by the Institute of Nuclear Medicine and Allied Sciences (INMAS) — a lab under the Defence Research and Development Organisation (DRDO) — in collaboration with Dr. Reddy’s Laboratories, Hyderabad.
What is 2DG?
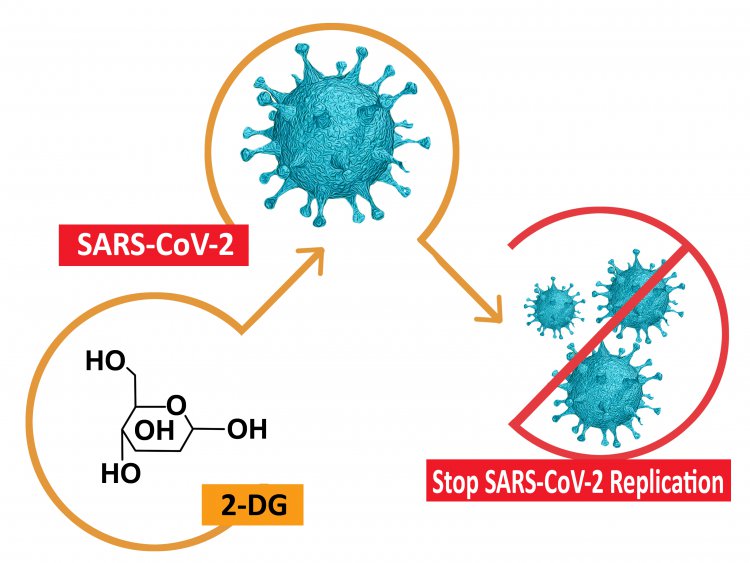
2-DG, short for 2-Deoxy-D-Glucose is a drug formulated by DRDO, which was found to be effective against SARS-CoV-2 virus and inhibited viral growth. 2-DG comes in powder form in a sachet and is to be taken orally after dissolving it in water.
How does it work?
Based on the results from the clinical trials, it has been found out that the drug helps in faster recovery of hospitalised patients and reduces supplemental oxygen dependence.
It works by gathering inside the cells infected by SARS CoV-2 virus and prevents further growth by halting any viral synthesis and energy production. The ministry said that “its selective accumulation in virally infected cells makes this drug unique". Trial results revealed that a higher proportion of patients treated with 2-DG were found to display “RT-PCR negative conversion" and that the “drug will be of immense benefit" to Covid-19 patients.
2DG’s Efficacy
In May 2020, DCGI's Central Drugs Standard Control Organization (CDSCO), permitted to conduct Phase-II clinical trials on Covid patients. Phase-II trials began by administering dosage of 2DG on 110 patients across the country between May and October 2020. At this stage, 2-DG was found to be safe and effective in Covid patients and reported to have shown significant improvement in their recovery.
Based on this encouraging data from the Phase-II trial, the DCGI further permitted the Phase-III clinical trials in November 2020. During the final stage trials between December 2020 and March 2021, 2-DG is said to have been administered to 220 patients across the country. At this stage, 42% of patients were free from supplemental oxygen dependence by Day 3 as compared to 31% of patients treated in accordance with the existing standard of care. The trend of early relief from oxygen dependence is reported to have been consistent in patients aged more than 65 years as well.
Availability
On May 1st, DCGI had cleared the formulation for emergency use as an adjunct therapy in moderate to severe Covid patients and the first batch of the homegrown anti-Covid drug was released on May 17th in the presence of Defence Minister Rajnath Singh and Health Minister Dr Harsh Vardhan.
2DG is expected to be rolled out by mid-June 2021. Price is determined by keeping an eye on making it affordable to all & will be announced soon.


 Admin
Admin